25% of patients with stable renal function are experiencing “silent” subclinical acute rejection that could lead to worse outcomes.
TruGraf® Kidney offers the Earliest Possible Detection of “Silent” Subclinical Acute Rejection

A serial testing strategy relying on TruGraf provides an attractive alternative for programs that do not routinely perform protocol biopsies, enabling them to stratify their stable patient population into those likely to be adequately immunosuppressed or not, without waiting for onset of clinical symptoms to guide therapy.
25% of Your Patients May Experience Silent Rejection. Do You Know Which Ones?
Despite improvement in short-term outcomes, long-term outcomes for kidney transplant recipients remain suboptimal. Immunological rejection is a leading cause of graft failure and recent research points to “silent” subclinical acute rejection (subAR) in patients with stable renal function as a key component of this problem. SubAR is termed “silent”, because it cannot be detected by current non-invasive standard of care tests, and requires an invasive tissue biopsy. 25% of stable kidney transplant patients are experiencing silent rejection—organ failure before observable symptoms of rejection appear—that lead to worse outcomes.
Previous Methods of Detecting Subacute Clinical Rejection Are Insufficient
Traditionally, a surveillance biopsy performed on a patient with stable renal function was the only way to identify or rule out the presence of subclinical acute rejection.
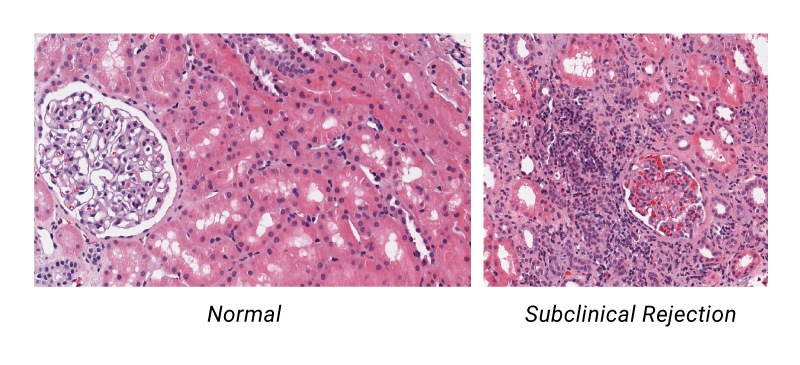
Post-transplant patient monitoring has typically involved testing for immunosuppressive drug levels which can only indicate whether patients are taking their medications as prescribed (or not), and measuring serum creatinine levels. While creatinine is an excellent indicator of renal function, it’s a late trailing indicator of damage already done to the transplanted kidney as 50% of kidney function may be lost before serum creatinine goes up.
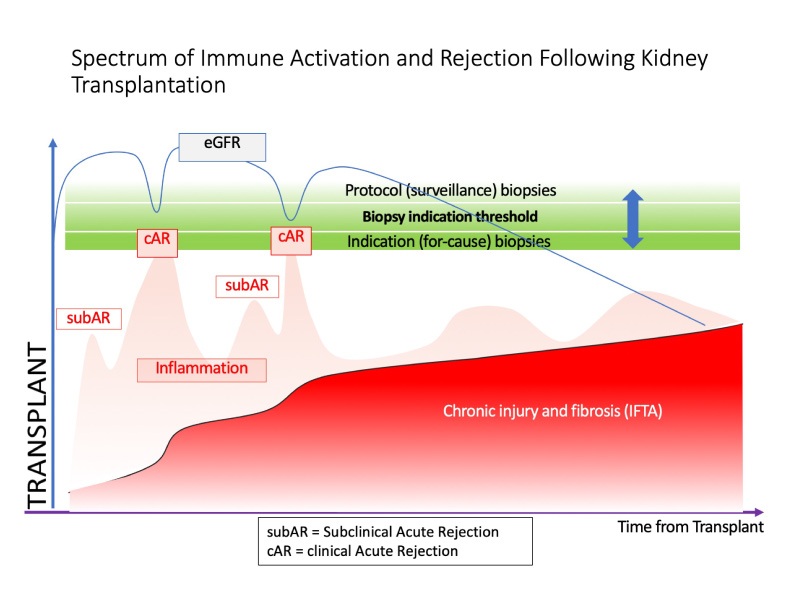
Early studies ignored the prevalence of borderline rejection in protocol biopsies. Recent studies demonstrated that:
- subclinical acute rejection is prevalent in 25% of patients; histology is characterized as borderline in 80% of these cases
- patients with subclinical acute rejection can develop chronic rejection, interstitial fibrosis and tubular atrophy (IFTA), and potentially graft loss
- patients with subclinical acute rejection have worse long-term outcomes, if untreated
It has been widely acknowledged that a non-invasive method that stratifies patients into high risk of rejection and low risk of rejection before allograft damage occurs would offer significant advantages to physicians for treating their patients, especially in terms of patient safety and graft longevity.
See What’s Behind TruGraf
TruGraf: the Alternative to Biopsies for the Earliest Detection of Silent Rejection
TruGraf is the first non-invasive blood test that offers clinicians the earliest possible guidance of whether their patients are adequately immunosuppressed, prior to a rise in their serum creatinine — when damage to the kidney has already occurred — without having to perform a risky and invasive biopsy.
TruGraf measures differentially-expressed genes in the blood of renal transplant recipients to identify patients who are likely to be adequately immunosuppressed, and in doing so rule out subclinical acute rejection.
TruGraf uses RNA microarray technology to determine whether a patient’s blood gene expression profile is more similar to that obtained from a reference population classified by simultaneous histological analysis of a biopsy as Transplant eXcellence (TX), and likely adequately immunosuppressed, or Not-TX, and likely to be inadequately immunosuppressed.
Key benefits of serial testing with TruGraf include:
- patients benefit by avoiding painful, risky, invasive procedures that are negative 75% of the time
- patients and their caregivers benefit from the knowledge that they don’t harbor silent subclinical rejection without having to undergo an invasive procedure
- transplant clinicians benefit by stratifying patients into those who may benefit from surveillance biopsy and those who likely won’t, allowing them to eliminate a large percentage of surveillance biopsies that would likely have been negative if performed
Indications & Contraindications for Use
The TruGraf test is intended for use in kidney transplant recipients with stable renal function as an alternative to surveillance biopsies.
Patients who meet all of the following criteria are eligible for TruGraf testing:
- Males or females of at least 18 years of age.
- Recipient of a primary or subsequent deceased-donor or living-donor kidney transplantation.
- Stable serum creatinine (current serum creatinine <2.3 mg/dl, <20% increase compared to the average of the previous 3 serum creatinine levels)
- Kidney transplant patients who are more than 90 days post-transplant
TruGraf has not yet been evaluated in patients who are:
- Recipients of a combined organ transplantation with an extra-renal organ and/or islet cell transplant.
- Recipients of previous non-renal solid organ and/or islet cell transplantation.
- Infected with HIV.
- Patients with BK nephropathy.
- Patients that have nephrotic proteinuria (urine protein >3 gm/day).
Limitations:
- The performance and clinical utility of TruGraf have been established in a cross sectional study (i.e., single point in time) at transplant centers utilizing surveillance biopsies. Clinical utility of TruGraf at centers not utilizing surveillance biopsies is currently being investigated.
- A TruGraf test result of TX does not guarantee adequate immunosuppression. Patients with a TX TruGraf test result should be advised to continue monitoring as per their local standard of care and clinical assessment.
- TruGraf may produce false negative or false positive results. A false positive result occurs when TruGraf produces a not-TX result, even though a biopsy will not find evidence of immune activation. A false negative result occurs when TruGraf produces a TX result, even though a biopsy will identify evidence of immune activation.